This year, the Phase 1 study of an mRNA HIV vaccine is starting, and the study has been published in the U.S National Library of Medicine. The HIV virus is still a public health concern worldwide, and over the past decades, researchers have been working hard to develop an effective vaccine against the infection.
An early-stage for the mRNA HIV vaccine is about to start
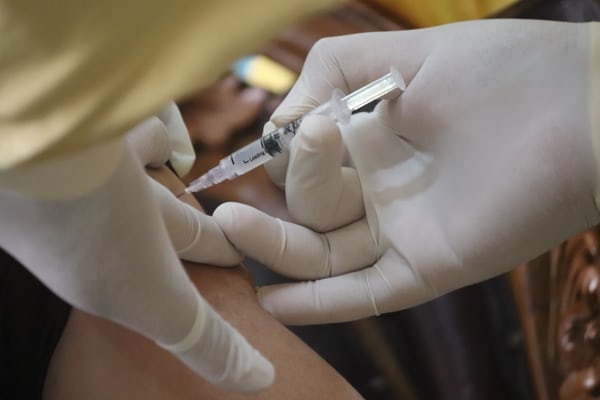
According to the study, the first early-stage human trial will evaluate the safety and immunogenity of mRNA-1644 and mRNA-1644v2-Core in HIV-1 uninfected adults. All participants selected for the study are in good health, and the vaccines should stimulate their immune systems to produce antibodies against multiple HIV variants. The stimulation should help the immune system recognize several HIV strains and prevent the infection from entering human cells.
Moderna’s mRNA technology will be used
The technology used for the vaccines is developed by the pharmaceutical biotech company Moderna, and it is the same one used for the Covid-19 virus. Similar to the SARS-CoV-2 virus, HIV has a spike protein, the Env, and due to its characteristics, it is hard to be eliminated by antibodies.
The study will have 56 participants, and it will begin on September 19 and end in April-May 2023. The collaboration between the International AIDS Vaccine Initiative and Scripps and Moderna could bring hope to eradicate the HIV.
The purpose of the study is to assess if the vaccine can effectively deliver the blueprint for making a specific protein to human cells. The human cells should be able to produce it and reject the HIV infection. The two vaccines will be administered differently to the participants. Some will receive a mix, while others will receive doses of one or the other. The results will take a while to be analyzed but it is the best option so far.
























Leave a Reply